The American Diabetes Association’s 82nd Scientific Sessions, were held from June 3-7 both in person and virtually. This annual conference brings together researchers and scientists to both present and learn about the latest in type 1 diabetes research and technological advancements. Many of the presenters are funded by JDRF International, JDRF Canada’s affiliate in the United States.
You can view all the oral and poster presentations on the Diabetes journal website.
Updates in cure-based research:
Stem Cell-Derived Beta Cell Therapy
Vertex, ViaCyte and Sernova are making exciting progress in the areas of stem cell derived beta cell therapy, and have all received JDRF funding at some point for their research.
Funding cell replacement therapies research is one of JDRF’s most critical undertakings globally, in its efforts to support the most promising cure-based research into type 1 diabetes (T1D).
Vertex launched its clinical trial of VX-880, a stem cell-derived beta cell therapy in T1D, in combination with immunosuppressive therapy to protect the cells from rejection, in the summer of 2021. To date, three participants have received the therapy, and one is now insulin independent. ViaCyte, in partnership with CRISPR Therapeutics, initiated its first-in-human gene-edited, stem cell replacement therapy, without immunosuppression, called VCTX210.
Sernova provided an update on the phase I/II clinical trial of their Cell Pouch™—an implantable device designed to form a natural environment and allow the survival and function of insulin-producing (islet) cells. The first three individuals to receive the therapy into the Cell Pouch™, with a supplemental marginal dose of islet cells via the portal vein, have been insulin independent for 2 years, 6 months, and 3 months, respectively. JDRF continues to support Sernova to make their Cell Pouch part of the cure portfolio.
There were also presentations on clinical and preclinical data from several investigations on encapsulation and immune-tolerance strategies, including JDRF-funded James Shapiro, M.D., Ph.D. and Andrew R. Pepper, Ph.D., of the University of Alberta, Canada.
Disease-Modifying Therapies
T1D is caused by adaptive immune cells attacking and eventually killing the beta cells in the pancreas that are responsible for producing insulin – but beta cell stress and dysfunction precede the complete loss of cell function, and immune cells are responsible. The prohormone to islet amyloid polypeptide (proIAPP, for short)—as C. Bruce Verchere, Ph.D., and Rebecca Hull-Meichle, Ph.D., discussed in their presentations—is elevated prior to clinical diagnosis, in addition to proinsulin—the precursor to insulin. proIAPP, in turn, causes inflammation and innate immune cell damage.
Dr. Verchere has developed a test to measure two kinds of proIAPP in humans, which will ultimately provide new insight into beta cell function and pave the way for new therapies and biomarkers of beta cell stress. What does this mean? Potential medications or disease modifying therapies could be brought to market that could slow or stop the immune response responsible for killing the beta cells.
Dr. Verchere received a JDRF postdoctoral fellowship beginning in 1992 and Dr. Hull-Meichle received one in 2001-2003.
Dr. Verchere is now leading the JDRF Center of Excellence at the University of British Columbia, where he is working on immune and beta cell therapies, including stem cell-derived therapies, with Dr. Kieffer; James Johnson, Ph.D., who received a JDRF Career Development Award in 2005-2010; Francis Lynn, Ph.D., who was a JDRF postdoc from 2004-2006, advanced postdoc from 2007-2009, and a JDRF Alan Permutt Career Investigator from 2012-2016; and Megan Levings, Ph.D., who has received two JDRF grants since 2015 and has been a mentor to two JDRF postdocs.
Type 1 Diabetes Screening in the General Population
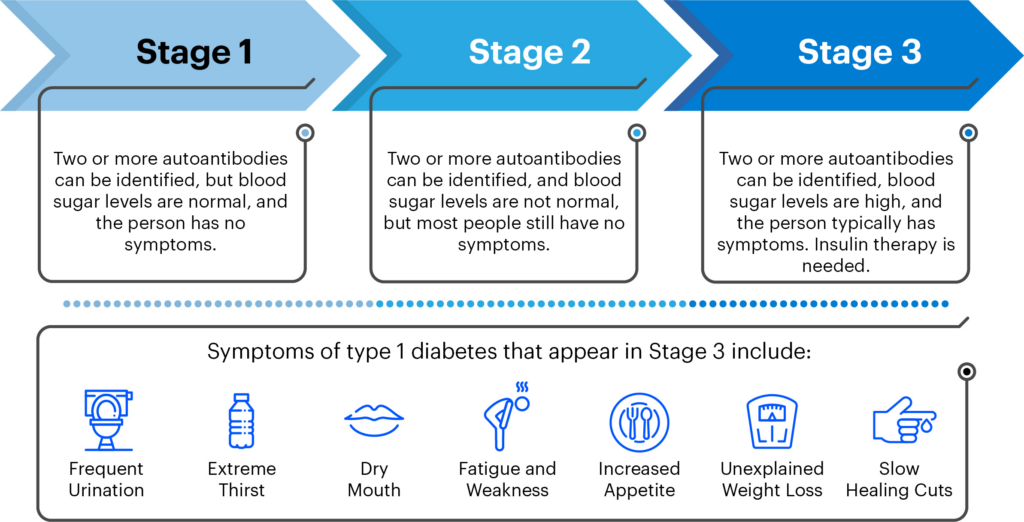
As a result of decades of JDRF-funded research, it is now possible to identify those at highest risk for developing T1D—those who have two or more autoantibodies present in people with type 1 diabetes.
Several JDRF-funded researchers presented on the current state of screening for both genetic risk and/or T1D-related autoantibodies. Chantal Mathieu, M.D., Ph.D., gave a talk about why it’s time to screen for T1D in the general population. She emphasized that T1D is a serious disease and that decreasing the incidence of diabetic ketoacidosis (DKA) a potentially life-threatening complication that is often the first sign of T1D in individuals – is cause to warrant population screening.
Post-screening, healthcare practitioners must be available for follow-up and guidance so families know what to do with autoantibody status, including potentially enrolling in clinical trials of disease-modifying therapies like teplizumab, a drug that may slow the development of T1D before DKA. Screening will help identify populations who can benefit from these therapies.
Screening is now a priority of the JDRF-CIHR Partnership to Defeat Diabetes, as it’s seen as a path to a cure by developing disease-modifying therapies to keep the disease from progressing and, ultimately, prevent it entirely.
Improving Lives
Artificial Pancreas Technologies
There were multiple presentations on the ‘artificial pancreas’, or automated insulin delivery (AID), systems.
Some presentations highlights included the results from the first randomized clinical trial testing of a do-it-yourself, or DIY, open-source, community-built AID technology, using the OpenAPS algorithm plus the DANA or YpsoPump insulin pump and the Dexcom G6 continuous glucose monitor (CGM).
The study included 100 children and adults in New Zealand who used the DIY system compared to those without the algorithm, headed by JDRF International grantees Martin de Bock, Ph.D. (who also gave the presentation), and Dana Lewis, the founder of the DIY artificial pancreas system movement. There was no severe hypoglycemia and no DKA, and more participants achieved time-in-range of ˃70% using the OpenAPS algorithm, especially at night.
As well, there was a presentation on the results from a randomized insulin-only iLet bionic pancreas pivotal trial. These were presented by Steven Russell, M.D., Ph.D., at the Advanced Technologies & Treatments for Diabetes (ATTD) conference in April 2022, but now we have reports from the participants, presented by Jill Weissberg-Benchell, Ph.D., a professor at the Ann & Robert H. Lurie Children’s Hospital of Chicago. The study included adult participants who reported decreased distress, with less burnout due to increased time-in-range and no need to carbohydrate count, and youth who reported positive experiences, including improved A1c, increased independence, and less time managing diabetes.
Diabetic Retinal Disease
Sobha Sivaprasad, M.D., reported on The Restoring Vision Moonshot, an approach to ending diabetic eye disease. The Early Treatment Diabetic Retinopathy Study (ETDRS) Scale was developed in the 1950s but was limited to point-in-time visual perception.
Dr. Sivaprasad is part of 50 global experts who will review the literature on diabetic eye disease in the next year, to develop an evidence-based updated retinopathy staging scale, creating recommendations that will incorporate decades of progress in functional imaging, other biomarkers, and metrics of quality of life. When this scale is completed, it will lead to the development of early preventive therapies to reduce vison-threatening retinopathy progression, and ultimately improve quality of life for people with T1D.
It’s an exciting time in type 1 diabetes research. JDRF will continue to monitor these studies and provide further updates as they become available.