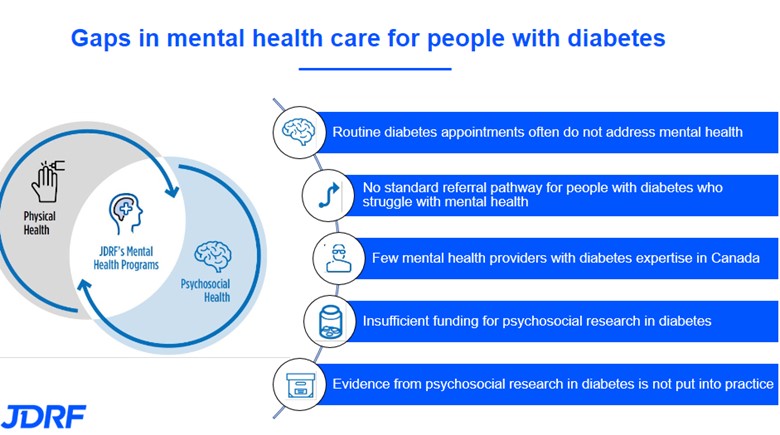
Alison Hunt is celebrating her 28th DiaVersary by walking 28km to raise funds for JDRF and T1D research
I was born and raised in Sudbury, Ontario and moved to Ottawa, Ontario in 2010 to start my undergraduate degree. I graduated with an Honours Bachelor of Social Sciences in International Development and Globalization from the University of Ottawa in 2014, and stayed in Ottawa where I currently reside with my husband J-F and our one-year-old Doberman, Rogue. In my spare time I enjoy travelling, going to sporting events and concerts, baking, playing board games with friends, and walking and snuggling with my dog!
A DiaVersary is the day that you were first diagnosed with type 1 diabetes (T1D). Can you tell us a little more about what you remember from that day and why you have chosen to make it a positive with this fundraiser?
I was diagnosed with T1D in August 1996, a couple of weeks before starting my first year of school. Having just turned four years old, I don’t remember much about my hospital stay when I was initially diagnosed. I do remember my older brother coming to visit me in the hospital and playing a Space Jam arcade game in the hospital’s playroom, and going for walks along Ramsey Lake where I painted rocks.
I also remember that by the time I left the hospital, I had quite the collection of finger puppets knit by hospital volunteers, which served as a welcome distraction from the many finger pricks (blood tests to test blood glucose levels)!
My mom learned to administer needles on an orange and counting carbohydrates was more challenging back then as not all food items had nutrition labels like most do now. Being an avid dog lover for as long as I can remember, one of my fondest post-diagnosis memories was receiving an enormous stuffed animal dog from the owner of my dad’s place of work, which I at first thought was real, and later named Mutsy.
Growing up with T1D I played numerous sports including soccer, tennis, rowing, dance, horseback riding, swimming, and badminton, enjoyed sleepovers and birthday parties with friends, and went on many school field trips and family trips. My family never let my T1D get in the way of enjoying all the things that a child without T1D would enjoy – it just became our new life routine, and I owe the positive attitude I have around T1D today to them. While it took a lot more planning, particularly around insulin dosing and carbohydrate counting, they never let that stop me from trying new things or making new memories.
What changes have you seen in T1D management since your first ‘DiaVersary’?
I was on multiple daily injections from 1996 until 2016 when I switched to an insulin pump (Animas and later Tandem) and not long after, started on a continuous glucose monitor (CGM) (Dexcom).
Having a CGM truly changed my life as I had developed hypoglycemia-related anxiety by adolescence, and, as a result, chose to miss out on activities as well as keep my blood glucose levels a bit higher than they should have been in an effort to prevent lows. I now have the best control I’ve ever had, but it is not solely because of these medical devices.
If I could teach the public one thing about T1D, I would want them to know that while diabetes technology has come a long way and has definitely facilitated some aspects of diabetes management, the onus for management still lies on the individual with T1D, and it is not as simple as counting carbohydrates and taking insulin.
I owe my great diabetes control to my unrelenting hard work, perseverance, and dedication, as do many other T1Ds – all things I have learned to take great pride in. While some days it’s easy to think, “Why me?”, I’m a firm believer in making the most of the cards you’ve been dealt. As a result, I’ve made the conscious decision to prioritize my health in order to ensure I have the lowest chance of developing complications from things that are within my control. I know that I am the most self-disciplined, patient, and resilient version of myself as a result of my diagnosis and for that I am very grateful.

What has fundraising for JDRF meant to you personally?
T1D is a very isolating condition – you are constantly counting carbohydrates, treating lows, reviewing your Dexcom graphs for patterns, bolusing (insulin administration) for food, correcting highs, planning and making adjustments for exercise, experiencing many sleep deprived nights, coping with the stress of it all…the list goes on. There are truly no breaks – it is on your mind 24/7, 365 days a year. At the same time, it is also a very invisible condition as you likely wouldn’t know someone has it unless you know what an insulin pump or continuous glucose monitor is, and you can visibly see it (or if you see an adult chugging a juice box and know what that means!!).
Fundraising for JDRF makes me feel incredibly loved and supported by all my friends and family who either donate, join my team and walk with me, or even reach out with a kind message of support. It always reminds me that I am not alone in this fight, however isolating it may feel at times, and for that I am so appreciative. I know that I am also greatly benefiting from the investments in research that are being made as a result of the funds raised.
In honour of my 25th “diaversary”, I decided to walk 25KM over two days, and did this again the following year, walking 26KM. I took the year off in 2023 as I was busy preparing for our wedding but am once again participating in the JDRF Walk to Cure Diabetes this year and will be walking 28KM over two days in honour of my 28th “diaversary” in August. I hope to continue doing this for many years to come.
What are your hopes for the future?
I am not certain that we will turn type one into type none in my lifetime, but I hope T1Ds like myself will continue to benefit from advances in technology – things such as being able to give a bolus from your cell phone (making dress-wearing a lot easier!), more advancements to closed-loop systems, and even smaller and longer lasting CGM sensors. I also hope that there will be increased public education around T1D, which is so commonly mistaken for T2D.
I spent many years feeling ashamed of my diagnosis and often tried to hide my condition because I never wanted to stand out. But as I grew older, I gained more confidence and my mindset changed from wanting to hide to wanting to educate and inspire others, and I hope that reading my story has done just that.
Alison is celebrating her DiaVersary through JDRF’s Fundraise Your Way program, that lets people fundraise in ways that are meaningful to them. To learn more about her fundraiser: https://jdrf.akaraisin.com/ui/jdrfwalk2024/p/ahuntT1D