Cell Therapy
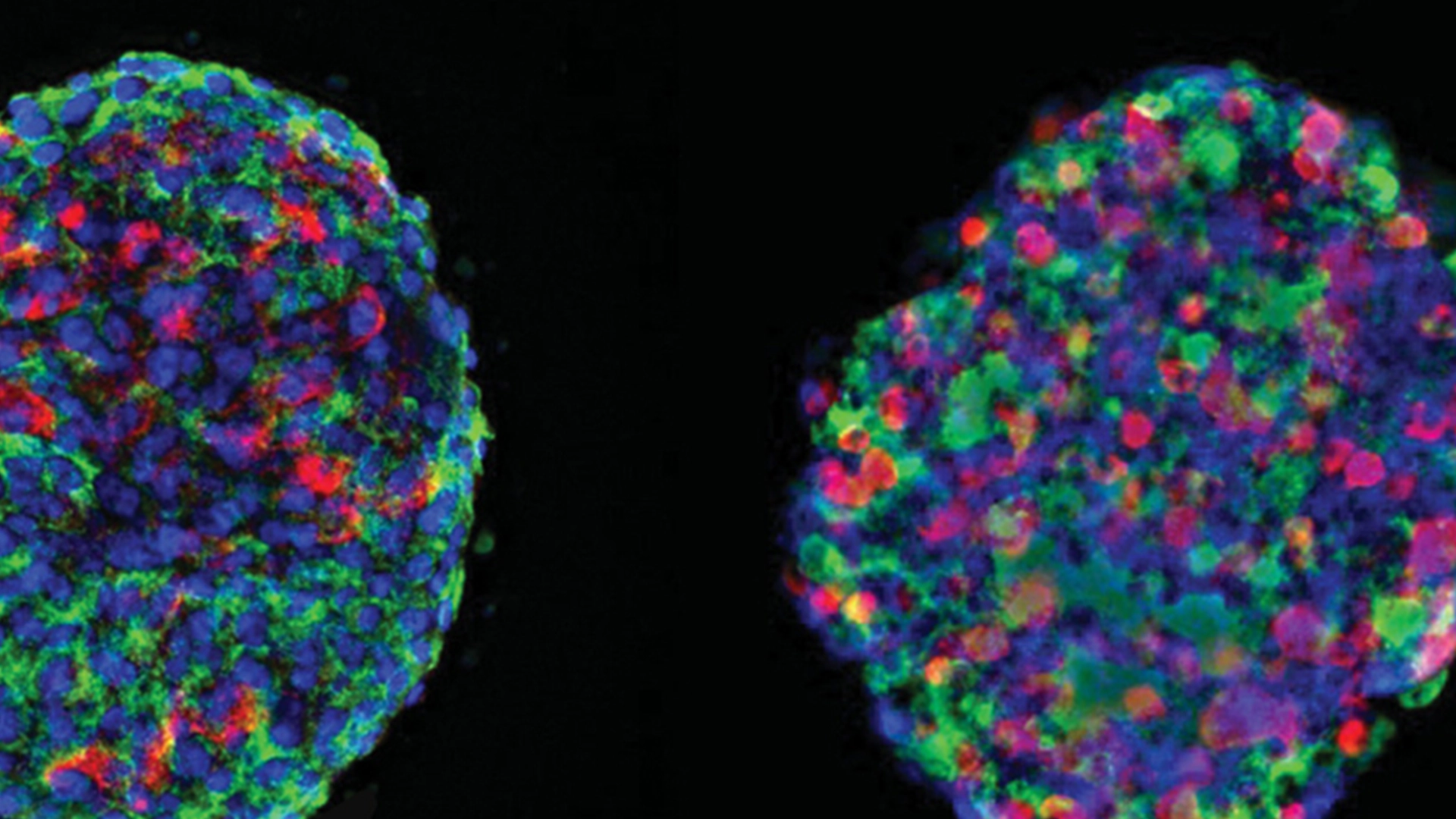
Curing type 1 diabetes (T1D) is the primary goal of Breakthrough T1D and many of the 300,000 Canadians living with T1D and their families. Cell therapy is one of the most active and fast-moving avenues of research in this area, but what is it and how close are these approaches to becoming a reality?
In the context of Breakthrough T1D’s global research strategy, cell therapy generally refers to the replacement (transplantation) of glucose-sensing, insulin-producing cells that are destroyed in a person with T1D.
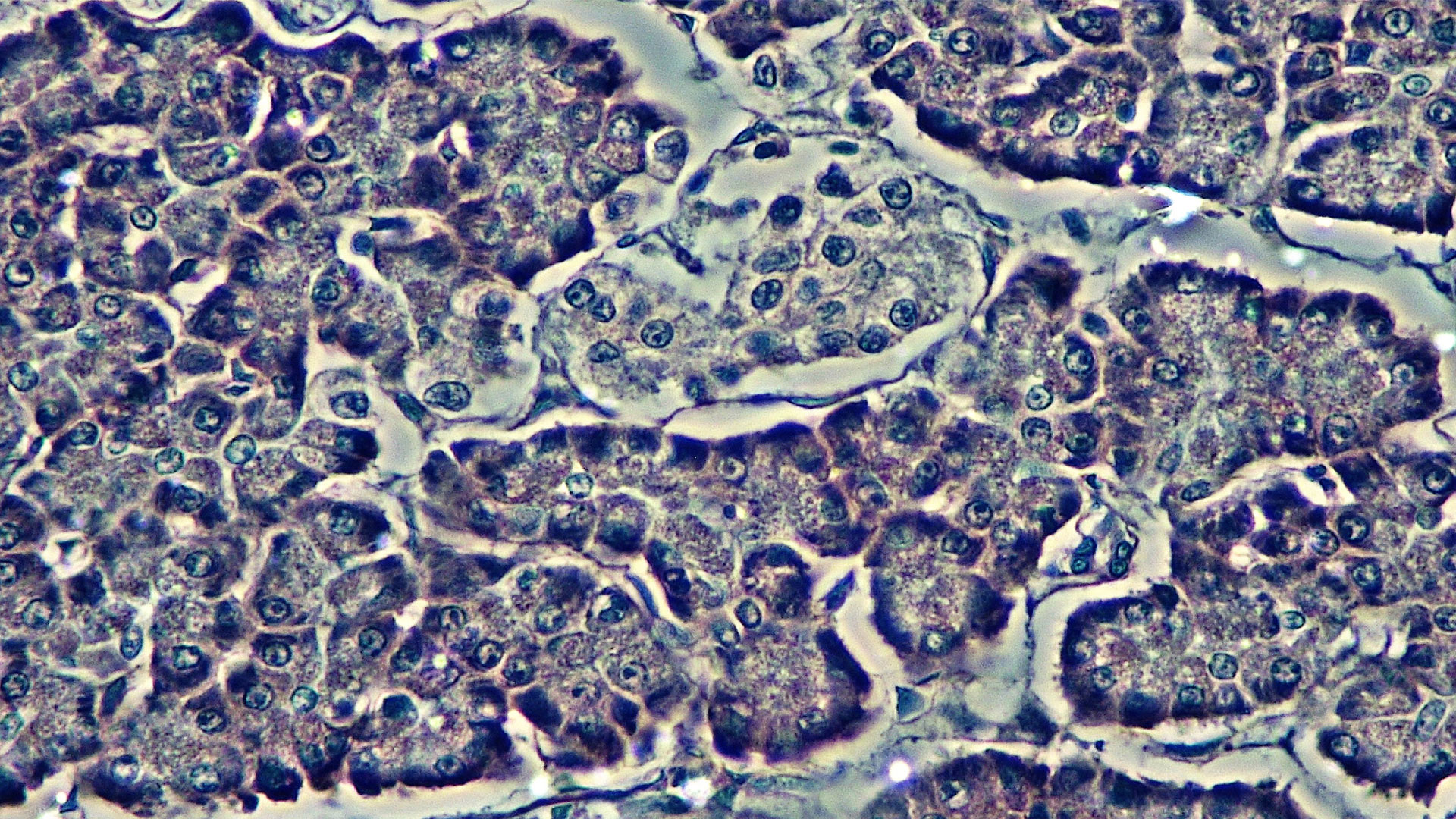
Islets are the clusters of cells within the pancreas that secrete hormones. They are comprised primarily of beta cells, which sense glucose levels and secrete insulin in response. Alpha, delta, and gamma cells also make up islet cells – these cell types also help with the nuances of blood sugar regulation by secreting hormones like glucagon and amylin.
Since 1999, islet replacement therapy has become more available in Canada since development of an approach by Dr. James Shapiro and his team at the University of Alberta named “The Edmonton Protocol”. The Edmonton Protocol involves infusion of islets isolated from the pancreas of deceased organ donors into the liver of a person with T1D. Almost 300 people in Canada and many more worldwide have undergone islet transplantation, under the auspices of both research studies and clinical procedures. However, most people don’t consider this to be a cure for T1D for many reasons. Most notably, results in Canada show that 5-years after having an islet transplant only 32% of people were still insulin-independent [1].
The main challenges with traditional islet replacement therapy are the need for lifelong immunosuppression, which is associated with side-effects and risks, and the need for multiple islet infusions over time due to the poor longevity of the cells. Logistically, another significant hurdle exists – the scarcity of islets required for the procedure. It usually takes 2-3 pancreases to extract enough cells for a single transplant, and many people need a “top up” of even more donor islets when the original transplant begins to fail. There will never be enough cells to cure T1D in Canada with donor islets. Therefore, the field was faced with the challenge of identifying an endless or renewable cell source to fill this gap. Enter, stem cells.
Stem cells were discovered in Canada by James Till and Ernest McCulloch in 1961. These cells are special because of something called pluripotency – the ability to develop into any cell type in the body if the right cues are provided. Since their discovery, the field of regenerative medicine has exploded. Specific to diabetes, research in the early 2000s began exploring the possibility of creating islet cells or beta cells from stem cells, and in 2014 two laboratories, separately but simultaneously, ‘cracked the code’. In the Fall of 2014, Dr. Doug Melton (Harvard) and Dr. Tim Kieffer (University of British Columbia) each published their work showing that insulin-producing beta cells could be generated from human stem cells in the lab. The work of these two groups largely paved the way for clinical trials now in progress by Vertex and ViaCyte (recently acquired by Vertex).
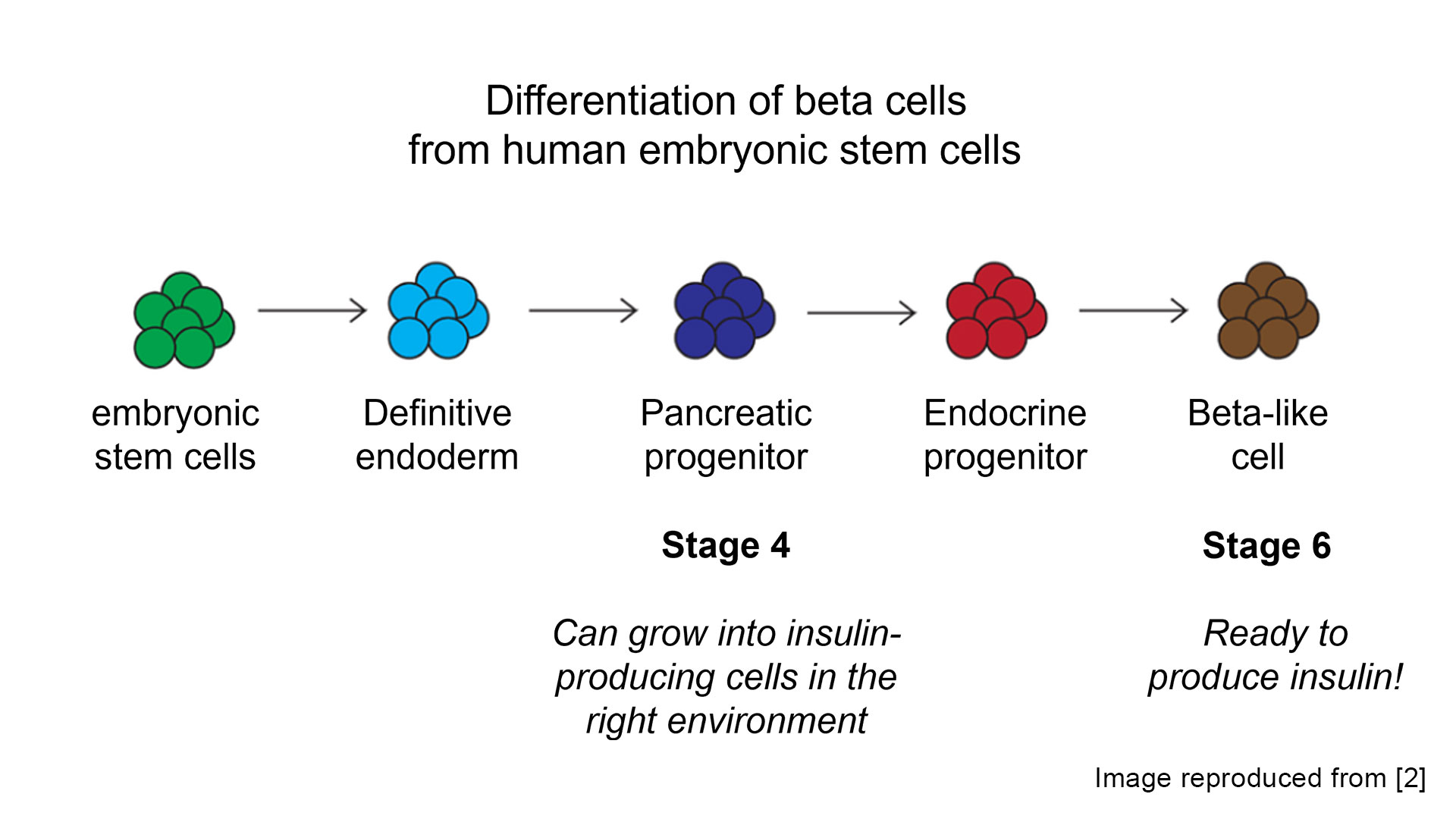
Cell source is still an ongoing area of research but optimizing the cell differentiation ‘recipe’ has benefited from years of research investment. Most research has focused on creating islet products from allogenic cells (meaning the cell source is obtained from a different person than will receive the treatment), which can allow for a scalable source of beta cells that would be used to treat many individuals. However, it is also possible to “re-program” a person’s own (autologous) cells back to stem cells that can then be differentiated to beta cells.
In September 2024, researchers from China reported the first transplant of beta cells created from a person’s own cells (in this case adipose (fat) tissue was used) in a twenty-five-year-old woman that was already receiving immunosuppressants for a liver transplant. Autologous cells may avoid or reduce the need for immunosuppression to reduce the risk of graft rejection since they are not from another donor. However, it is unknown if immunosuppression would still be required to prevent recurrent autoimmune attack associated with T1D. Breakthrough T1D is investing in this approach via a research grant to Dr. James Shapiro at the University of Alberta to address these scientifically important questions. However, Breakthrough T1D’s global research strategy in the area of cell therapies is focused on allogenic cells (meaning derived from another person’s cells) to create islet products that can be used to treat large numbers of people with T1D, rather than pursuing personalized therapies. The ultimate goal would be for these to be used without the need for immunosuppression.
References
- Marfil-Garza, B. A., Imes, S., Verhoeff, K., Hefler, J., Lam, A., Dajani, K., … & Shapiro, A. J. (2022). Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. The lancet Diabetes & endocrinology, 10(7), 519-532.
- Photiadis, Sara J.; Gologorsky, Rebecca C.; Sarode, Deepika. (2021) The Current Status of Bioartificial Pancreas Devices. ASAIO Journal 67(4), 370-381. 10.1097/MAT.0000000000001252
In the past decade, Breakthrough T1D has invested over $140 million USD internationally in advancing cell therapies towards the clinic. Despite immense progress, significant scientific challenges remain that are being addressed by Breakthrough T1D-funded work.
Scale-up & manufacturing: currently, the differentiation of stem cells into islet-like cells is only happening in very small quantities. This process will need to be greatly scaled up and automated to make cell therapy cost effective and available to a large number of people.
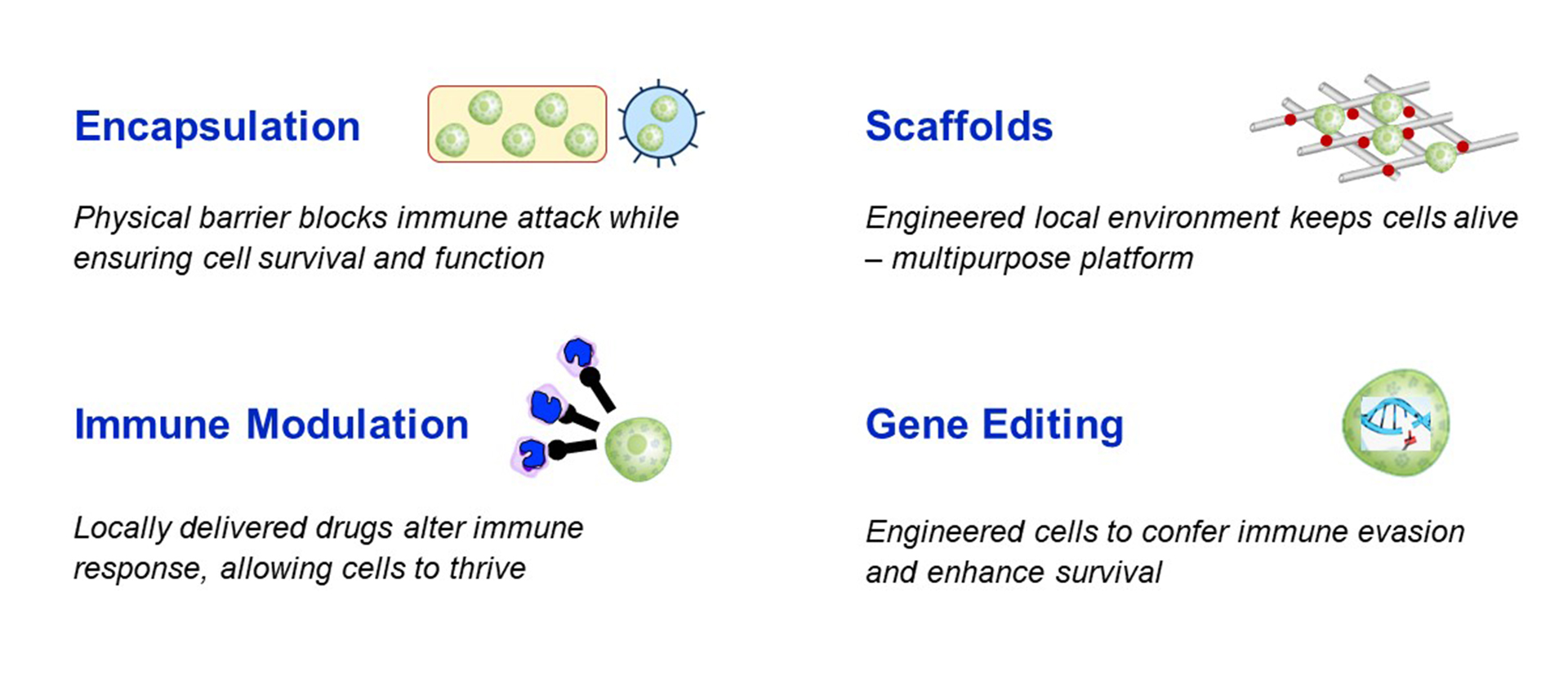
Immune protection: the most significant hurdle to cell therapy is the immune response – which is two-fold because not only is the human body programmed to reject foreign substances, but people with T1D are also programmed to directly attack beta cells. Here there are a few options that are being explored to avoid the need for whole-body (systemic) immunosuppression with islet replacement therapy:
-
- Immune modulation targets the immune system directly. This could either be local immunosuppression that will alter the immune response only for the beta cells, or a re-training of the immune system to learn not to attack the transplanted cells.
- Gene editing of the stem cells used to manufacture replacement islets is also being explored as an approach for immune protection or evasion. This can be done by modifying the genes that code for proteins on the cell surface that are recognized by the immune system, thereby making it harder for the immune system to detect and attack the transplanted cells.
- Encapsulation refers to a physical barrier made out of biomaterials to protect transplanted cells. Macroencapsulation (a barrier around all cells, like a teabag) or microencapsulation (a barrier around individual cells) can be made from immunoprotective materials which would physically shield the cells from being recognized and attacked from the immune system. A challenge of encapsulation is allowing the cells to survive (oxygen and nutrients must get into the device) and function (glucose must get in and insulin must get out of the device) with the protective device or coating surrounding them.
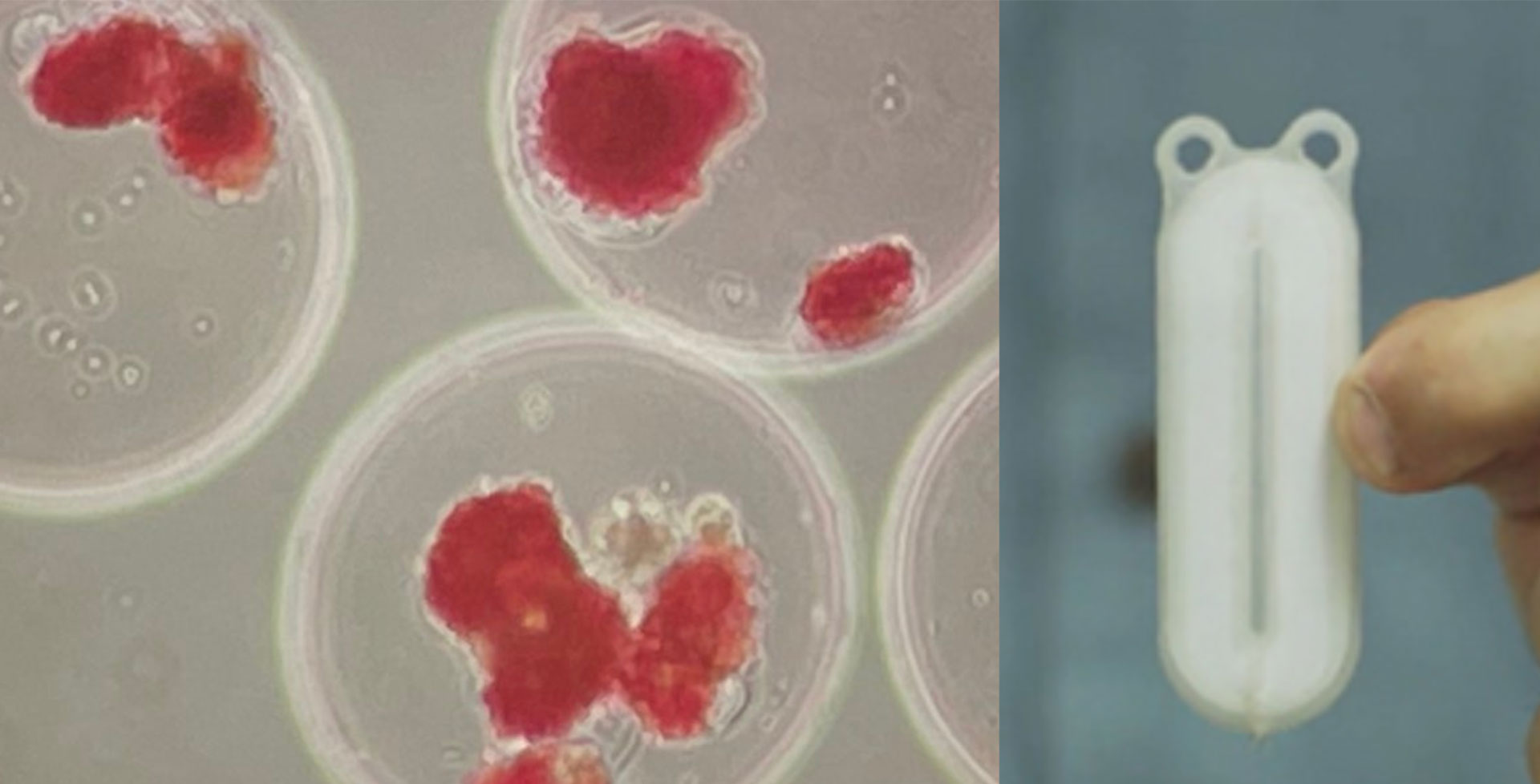
Retrievability of transplanted cells: The removal of cells is virtually impossible with islet infusion into the portal liver vein. The reason why this is important is that even once stem cells have been differentiated to islet cells, it does not mean that they will never change again. There is a small risk with any cell (but more so with a stem cell-derived cell) to become another cell type, including a cancerous cell. This is one of the arguments for macroencapsulation within a ‘device’ (like tea leaves within a teabag) since this would allow the entire group(s) of cells to be retrieved if there were any concerns.
Improving survival and engraftment: the survival of foreign cells in the body requires an immediate blood supply for the cells to receive vital nutrients. Successful engraftment occurs when cells survive transplant and begin functioning as intended (in this case, producing insulin in response to glucose). An important step for this is vascularization of the transplant, or connection with blood vessels. Currently, the immediate survival rate and engraftment rates are fairly low, which is why islet transplants have to be repeated over the years to add new cells in the place of previous transplant cells that did not survive. Strategies being explored to improve survival and engraftment include biological “scaffolds”, gene editing to over-express pro-survival genes, or treating cells or transplant recipients with drugs to boost their survival and engraftment.
Transplant location: current clinical practice for donor-obtained islet transplantation involves an infusion of the islets into the portal vein of the liver. Surprisingly, the pancreas is not a very hospitable environment to house islet cells, and therefore is not an option for optimal cell survival. Macroencapsulation (or devices) have been implanted into the subcutaneous space under the skin, typically in the arm and/or abdomen. This surface-level location allows for very easy retrievability but is less optimal for blood flow to get necessary nutrients to the cells. Other areas being explored, including the omentum – a large fatty tissue layer that lines the surface of the abdominal organs – and muscle. More research is needed to understand the ideal location for cells to function long-term.
So how do we move these cell therapies from the laboratory to clinical use? As with any new therapy – clinical trials are the crucial step to move research from lab bench to bedside. In the case of stem cell-based therapies, clinical trials in progress are being led by companies. However, much of the research, development, and optimization of these therapies is happening in academic research, with increasing academic-industry engagement.
ViaCyte was the first company to ever conduct a trial of a stem cell-based therapy for people living with T1D with its VC-01 stem cell-derived cells encapsulated within a device (i.e., a pouch to contain the cells) in 2014. Unfortunately, the initial trials showed significant limitations of the device itself. While later iterations (VC-02) demonstrated that the participants were producing C-peptide (a marker to indicate that insulin is being made within the body), it was not significant enough to reduce the need for external sources of insulin. In 2022, Vertex acquired ViaCyte – however, ViaCyte still operates as somewhat of a separate entity within Vertex, particularly since the ViaCyte trials are using different cells than the Vertex products. ViaCyte, in partnership with CRISPR Therapeutics, is also the first company to test gene edited cells (via CRISPR/Cas9 editing technology) for islet cell replacement. The products being tested in these trials (VCTX210 and VCTX211) have been edited to evade the immune response and are being tested for the safety, tolerability, and immune evasion in patients with T1D. This particular approach (i.e., gene edited stem cell-derived islet cells without the need for immunosupression) is considered the ‘ultimate goal’ for cell therapy by many.

Vertex Pharmaceuticals is particularly active in cell therapy for T1D and as of Spring 2024 is running two international clinical trials (VX-880 and VX-264) for people with T1D, not including the ongoing ViaCyte trials. Vertex has numerous significant investments in cell therapy including a 2023 collaboration with Lonza (a bio-manufacturing company) to build a dedicated manufacturing facility in the US, and a 2024 collaboration with TreeFrog Therapeutics to license TreeFrog’s proprietary technology platform C-Stem™ for large-scale cell manufacturing.
Other notable companies in this space include Sernova (based out of London, Ontario) which is testing their Cell Pouch System™ – an implantable medical ‘device’ that houses islet cells (see macro-encapsulation explanation above). Reports to date have been positive, that the cells within the device are surviving and producing insulin, however, it is important to note that all trials for the Sernova device have thus far been completed using cadaveric donor islet cells, not stem cells. Ongoing clinical trials are being conducted in Chicago, IL. In 2022, Sernova and Evotec entered an exclusive partnership to combine Sernova’s Cell Pouch with Evotec’s stem cell-derived beta cells. Sernova is also collaborating with Dr. Alice Tomei (University of Miami) to develop a microencapsulation (individual cell coating) with immune protection technology.
There are many other companies at a pre-clinical stage of development – too many to note them all. In Canada, Aspect Biosystems (based out of Vancouver, BC) was previously funded by Breakthrough T1D for the development of 3D-printed macro-encapsulation device that uses immunoprotective materials. Aspect Biosystems was acquired by Novo Nordisk in 2023 for continued development of this product to be used in conjunction with replacement islet cells being developed by Novo Nordisk. No trials are currently ongoing, but we will keep a close eye on this partnership. Allarta Life Sciences (based out of Hamilton, ON) was awarded a Breakthrough T1D Industry Discovery and Development Partnership grant in 2023 to advance their research into polymers and hydrogels (a material similar to what is used for contact lenses) that can provide an immunoprotective coating to islet-like cells.
Here is an abbreviated list of other companies around the world at pre-clinical stages of T1D cell therapy development (note that this list is not exhaustive, and information is current at time of writing):
- Eli Lilly partnered with Sigilon Therapeutics in 2018 and subsequently acquired Sigilon in 2023 to advance research into encapsulated cell therapies.
- Novo Nordisk has partnered with multiple companies (including Aspect Biosystems) to advance their cell therapy product.
- Sana Biotechnology who reported in early 2024 that their gene-edited, stem cell-derived islet-like cells effectively controlled blood sugar in a diabetic primate. With human clinical trials reported to be on the horizon for late 2024, we will watch this company closely.
- Eledon Pharmaceuticals are conducting clinical trials in Chicago, IL for use of their drug tegoprubart in combination with cadaveric donor islet transplantation to prevent rejection.
- Adocia is preparing for a first-in-human clinical trial expected end of 2024 / early 2025 for their AdoShell® Islets – an implantable and fully retrievable scaffold for islet transplantation that eliminates the need for immunosuppressive drugs.
- Betalin Therapeutics is focusing on development of a ‘micro pancreas’ using a scaffold developed of lung tissues.
- iTolerance, Inc. partnered with Kadimastem to combine immunomodulatory technologies with islet-like cells.
Lastly, there is the small but mighty presence of academic-led clinical trials in this space. Particularly, Dr. James Shapiro (University of Alberta and the pioneer behind the Edmonton Protocol) maintains an active research program heavily funded by Breakthrough T1D, while also being the surgical head of the most active islet transplant program in the world. Dr. Shapiro is investigating a variety of ways to reduce the need for immunosuppression with the current Edmonton protocol, and to develop a renewable source of islet cells using a person’s own cells. This last approach is not yet in clinical trials, but will aim to reprogram an individual’s own blood cells ‘back’ to a stem cell before differentiating them into islet-like cells appropriate for transplantation without immunosupression.
Table of Cell Therapies at a clinical development stage in Canada
| Cell source | Cell Protection | Cell Location | Availability / Location | |
|---|---|---|---|---|
| CLINICAL PRACTICE | ||||
| Islet Transplantation via the Edmonton Protocol *approved clinical therapy in Canada | Donor cells | Systemic immunosuppression | Infusion into portal vein of liver | In practice around the world since 2000 (*the US just approved Lantidra to use this protocol outside a research setting) |
| CLINICAL TRAILS | ||||
| CRISPR VCTX210 (Previously partnered with ViaCyte) | Stem Cells | Gene editing | Macroencapsulation (device) | Completed trials with no published results or information |
| CRISPR CTX211 (Previously partnered with ViaCyte) | Stem Cells | Gene editing (additional edits from VCTX210) | Macroencapsulation (device) | Ongoing trials in Canada (Vancouver, Edmonton) |
| Vertex VX-880 | Stem cells | Systemic immunosuppression | Infusion into portal vein of liver | Ongoing trials around the world (CA: Vancouver, Edmonton, Toronto) |
| Vertex VX-264 | Stem Cells | Macro-encapsulation | Macroencapsulation (device) | Ongoing trials around the world (CA: Vancouver, Edmonton, Toronto, Montreal) |
| Encellin Encapsulated Cell Replacement Therapy (EnCRT) | Donor Cells | Macro-encapsulation | Macroencapsulation(device) | Ongoing trials in Canada (Montreal) |
| University of Alberta (Dr. Shapiro) – Device-less Technique | Donor Cells | Systemic immunosuppression | Device-less implantation under skin | Ongoing trials in Canada (Edmonton) |
| University of Alberta (Dr. Shapiro) – PKX-001 | Donor Cells | Minimized immunosuppression via cell immune modulation | Infusion into portal vein of liver | Ongoing trials in Canada (Edmonton) |
-
1980
Researchers and clinicians demonstrate successful islet transplantation in 10 people that developed diabetes after removal of their diseased pancreas (pancreatitis), where the person’s own healthy islets were infused back after islet isolation. Ultimately, 3 of these people achieved insulin independence for 1, 9, and 38 months, respectively. 1
-
1987
A monoclonal antibody, which recognizes a specific cell type of the immune system, is used to transplant insulin-producing islet cells into mice, without relying on continuous use of potentially harmful immunosuppressive drugs.2 It is shown that rejection is prevented indefinitely in animal models.
-
1990
Two pioneering studies of islet transplantation in people with diabetes are performed on a total of 10 people in the US. The patients gained insulin-independence for a few weeks or minimized insulin need. These are the first unequivocal examples of successful clinical islet cell transplantation.3,4
-
1994
First-in-human trials of transplantation with pig (porcine) islets were done in people with type 1 diabetes and kidney failure. These studies were remarkable as insulin production from the islets was detectable for more than 300 days in many, and no serious side effects were observed. No reduction in insulin requirement and no insulin independence, however, was ever observed.5
-
1999
Dr. James Shapiro pioneers the Edmonton Protocol, using more islet cells and a less-toxic combination of immunosuppressive drugs than previous islet transplants.6 This is hailed around the world as an extraordinary breakthrough, and proof of principle that islet transplantation can potentially work and warrants further study. It remains the gold standard to date.
-
2000
Investigators at the Breakthrough T1D Center for Islet Transplantation (then JDRF) (including Dr. Doug Melton) demonstrate that the addition of certain growth factors could direct the development of human stem cells, setting the stage for the development of beta cells from stem cells in the laboratory.7 Breakthrough T1D funds a 10-year grant to Douglas Melton, Ph.D., for almost $1.4 million, to make beta cells from stem cells.
-
2001
Breakthrough T1D and the National Institutes of Health (NIH) co-found the Immune Tolerance Network (ITN), a seven-year, $144 million project, to conduct clinical trials aimed at inducing immune tolerance in the context of transplantation, autoimmune diseases, asthma, or allergies. The first major trial is a multicenter study to replicate and confirm the preliminary success of the Edmonton Protocol at 10 sites in North America and Europe.
-
2006
Novocell (later to become ViaCyte) develops a way to convert stem cells into insulin-producing cells.8 The cells do not secrete much insulin and don’t do so in response to glucose, but the research represents a vital first step in coaxing stem cells to become cells that sense blood sugar and ultimately produce insulin in response.
-
2011
Breakthrough T1D supports ViaCyte (formerly Novocell), which is developing an encapsulated beta cell replacement therapy that combines precursor beta cells made from stem cells that, over time, develop into mature pancreatic hormone-producing cells, including insulin-producing cells. Breakthrough T1D has been a significant supporter of ViaCyte since this time.
-
2013
Breakthrough T1D launches the Encapsulation Consortium, including researchers with expertise in bioengineering, beta cell biology, pancreatic transplants, and materials science, who will work to develop and incorporate new engineering concepts and designs that will improve islet cell encapsulation. Breakthrough T1D continues to convene this consortium today, now known as the Beta Cell Replacement Consortium.
-
2014
Significant progress is made in making beta cells in the lab: Two Breakthrough T1D-funded investigators (Dr. Timothy Kieffer, UBC & Dr. Doug Melton, Harvard University) develop a method for converting stem cells into insulin-producing beta cells.9, 10
—
With Breakthrough T1D support, ViaCyte starts its clinical trial of the first ever stem cell-derived encapsulated cell replacement therapy, VC-01. The trial will enroll approximately 60 people at multiple clinical sites.
-
2015
Dr. Doug Melton founds Semma Therapeutics to develop stem cell-derived beta cells into curative therapies for T1D. In 2017, the Breakthrough T1D Fund makes a catalytic investment in Semma to bridge the company to its next large venture round.
-
2019
Vertex Pharmaceuticals acquires Semma Therapeutics for nearly $1 billion.
—
ViaCyte reports on its VC-01™ therapy using stem cell-derived islet-like cells. Preliminary data show that when pancreatic precursor cells, called “PEC-01 cells,” are implanted under the skin and properly engrafted, they are capable of producing circulating C-peptide, a biomarker for insulin, in people with T1D.
—
Sernova, a clinical-stage regenerative medicine company, showed in its Breakthrough T1D-funded clinical trial that its Cell Pouch System™ allows for the production and release of insulin from the encapsulated cadaveric donor islet cells in people with T1D.
-
2020
Breakthrough T1D-funded researchers partner with the Advanced Regenerative Manufacturing Institute (ARMI) to translate the manual process of creating stem cell-derived beta cells into a reproducible and automated procedure so that sufficient numbers of quality-controlled islets could be distributed to the scientific community for research purposes.
-
2021
Breakthrough T1D Centre of Excellence (CoE) at the University of British-Columbia is launched in Vancouver. With a focus on immune and beta cell therapies, this is the first Breakthrough T1D CoE in Canada, joining a group of 4 other Centres in the US and Australia. The Breakthrough T1D CoE at UBC will accelerate cell therapy through three integrated themes: (1) developing a new source of insulin-producing beta cells, (2) protecting beta cells from immune attack, and (3) targeting and monitoring beta cell stress.
—
Vertex launches the clinical trial of VX-880, which uses stem cell-derived beta cells (pioneered by Dr. Doug Melton, Harvard University) to try to restore the body’s ability to produce insulin, in combination with immunosuppressive therapy to protect the cells from rejection, for people with T1D with severe low blood-sugar (called hypoglycemia) events and those who struggle to perceive the onset of hypoglycemia. Vertex announces promising data within the year, that the first person to receive VX-880, needs 91% less insulin after receiving an infusion of fully differentiated cells at just half the target dose.
—
A new first for ViaCyte and CRISPR Therapeutics: Gene-editing for T1D. By the end of the year, they will start a clinical trial of VCTX210, a gene-edited stem cell replacement therapy for this disease. Combining ViaCyte’s leading stem cell capabilities, which were developed with significant support from Breakthrough T1D, with CRISPR Therapeutics’ pre-eminent gene-editing platform, has significant potential in the development of a cell replacement therapy that does not require immune suppression, advancing ViaCyte’s mission of providing a cure for diabetes and Breakthrough T1D’s vision of a world without T1D.
-
2022
Vertex Pharmaceuticals announces that they have acquired ViaCyte for $320 million USD. Vertex and ViaCyte have been working independently to develop cell replacement therapies for people with T1D. This merger brings two of the biggest companies pursuing this technology for diabetes together, and will allow them to combine their resources, technologies, intellectual property, and more.
-
2023
CellTrans’s Lantidra™ is the first cell therapy to be authorized in the United States, for use in adults unable to approach average blood glucose levels due to current, repeated episodes of severe low blood glucose (hypoglycemia). This is a commercialization of cadaveric donor cell transplantation, which has been in clinical use in Canada since 2001.
—
Vertex reports VX-880 preliminary results: all six patients are self-secreting insulin, improved HbA1c levels, improved time-in-range on continuous glucose monitoring, and reduction or elimination of exogenous insulin use (i.e., externally administered insulin either by pen, pump or multiple daily injection). Patients with greater than 90 days of follow-up also had elimination of severe hypoglycemic events.
—
Vertex begins clinical trial of VX-264, which uses the same cells as VX-880 but encapsulated in an immunoprotective device. Due to the device, this therapy does not require the need for immunosuppression.
—
Sernova reports on its clinical trial (in Chicago, IL) that five patients had received an 8-channel pouch between 6-36 months previously. They have five patients enrolled to receive a 10-channel pouch which significantly increases the number of islets (from a cadaveric donor source) that will be transplanted in their Cell Pouch System.
-
2024
Vertex reports VX-880 preliminary results: Of the 12 patients who have been dosed, nearly all (11 of 12) have had a reduction or elimination of exogenous insulin use (via pump or injection). All patients have achieved an HbA1C below 7.0% and time-in-range above 70% on continuous glucose monitoring with the reduced or eliminated insulin administration. There have been no serious adverse events reported. The trial is expanding recruitment for 37 participants to progress towards pivotal development.
—
Sernova reports from their ongoing clinical trial (donor islet cells in Sernova’s Cell Pouch System; Chicago, IL) that the first 7 patients have achieved insulin independence. Enrollment is now completed for Cohort 2.
—
Vertex reports an update on the status of the VX-264 (UPWARD) trial: Part A of the trial is complete. This was a small number of participants who received a partial “dose” of the transplanted cells. These participants were staggered, meaning that there was a delay before each additional participant started the trial to ensure that the participant before them was tolerating the dose well. VX-264 is now recruiting and dosing participants in Part B of the trial (full dosing, meaning they get the full number of cells transplanted in a staggered start).
—
In September 2024, researchers from China reported the first transplant of beta cells created from a person’s own cells (autologous cells) in a twenty-five-year-old woman who was already receiving immunosuppressants for a liver transplant.
—
On November 4th, 2024, Vertex Pharmaceuticals made an important announcement that their Phase 1/2 clinical trial for their stem cell therapy VX-880 is converting into a Phase 1/2/3 pivotal trial following successful Phase 2 review by regulatory bodies. A pivotal trial gathers the data required for a regulatory submission (to Health Canada or other regulators such as the FDA) to bring the therapy to the market. This will build upon the ongoing international trial (including 4 Canadian sites) and increase the number of participants from 37 to 50.
-
2025
On Jan 7, 2025 (Sweden), Sana Biotechnology released significant clinical data: the first person with T1D who received deceased donor islets engineered to evade the immune system is producing insulin without immunosuppression at the 4-week follow up timepoint.
—
On Friday, March 28, 2025 news from Vertex came announcing that they are discontinuing one of their T1D cell therapy trials. VX-264 is the trial of stem cell-derived islet cells that are transplanted in a macroencapsulation device (like tea leaves in a teabag) without the need for immunosuppression. Unfortunately, although the first phase of the trial was safe and well-tolerated it was not effective and therefore won’t be continued.
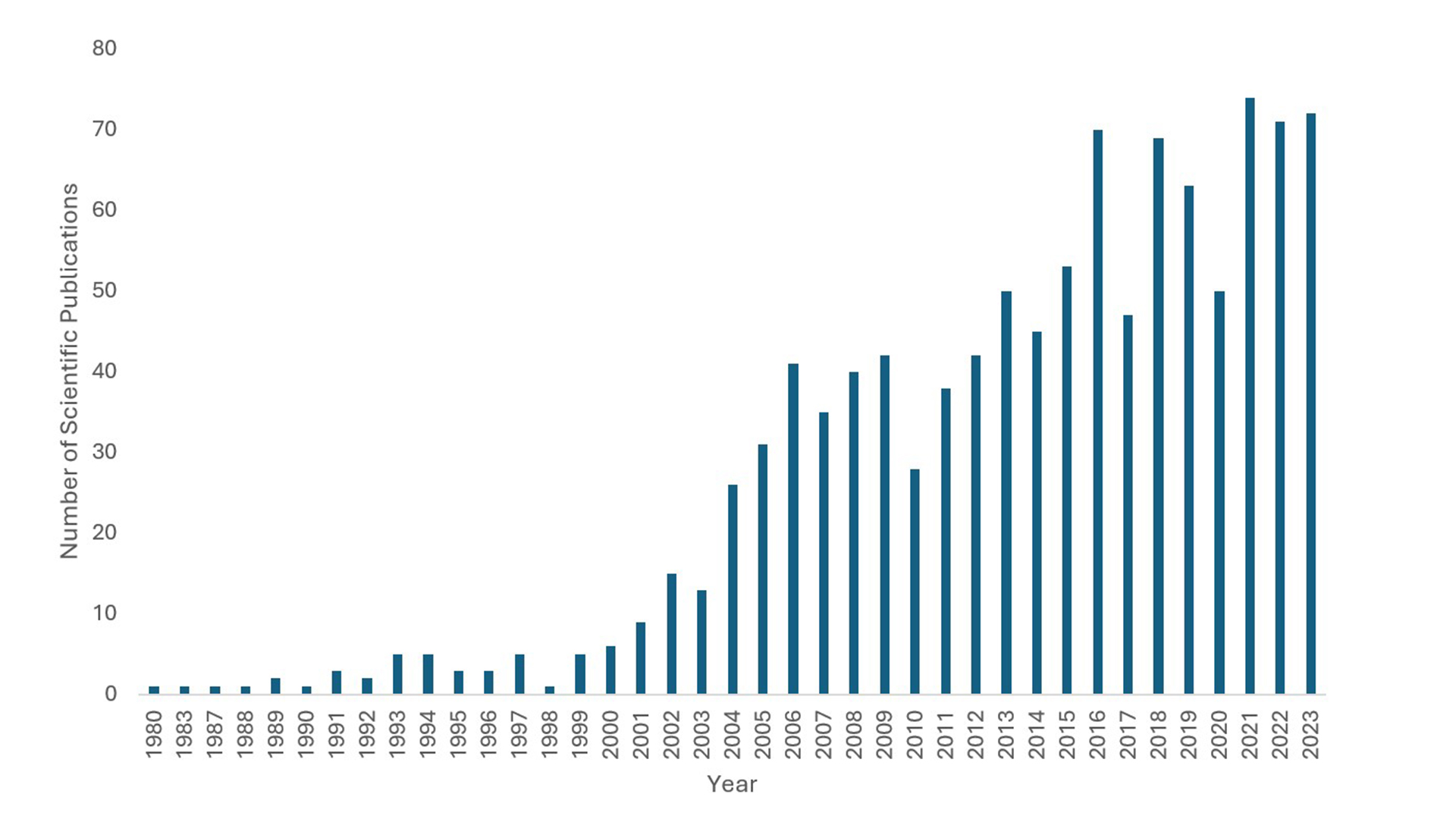
An overview of the increasing number of scientific publications in type 1 diabetes cellular therapy from 1980 onwards
References
-
-
- Najarian JS, Sutherland DE, Baumgartner D, Burke B, Rynasiewicz JJ, Matas AJ, Goetz FC. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg. 1980; 192 (4): 526-542. https://doi.org/10.1097/00000658-198010000-00011
- Shizuru JA, Gregory AK, Chao CT, Fathman CG. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987; 237 (4812): 278-280. https://doi.org/10.1126/science.2955518
- Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Falqui L, Marchetti P, Gingerich RL, Jaffe AS, Cryer PE, Anderson CB, Flye MW. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990 Apr; 39 (4): 515-8. https://doi.org/10.2337/diab.39.4.515 . PMID: 2108071.
- Tzakis AG, Ricordi C, Alejandro R, Zeng Y, Fung JJ, Todo S, Demetris AJ, Mintz DH, Starzl TE. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990; 336 (8712): 402-405. https://doi.org/10.1016/0140-6736(90)91946-8
- Groth CG, Korsgren O, Tibell A, Tollemar J, Möller E, Bolinder J, Ostman J, Reinholt FP, Hellerström C, Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994 Nov 19; 344 (8934): 1402-4. https://doi.org/10.1016/s0140-6736(94)90570-3. PMID: 7968077.
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343 (4): 230-238. https://doi.org/10.1056/NEJM200007273430401
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000; 97 (21): 11307-11312. https://doi.org/10.1073/pnas.97.21.11307
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006 Nov; 24 (11): 1392-401. https://doi.org/10.1038/nbt1259
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014 Nov; 32 (11): 1121-33. https://doi.org/10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014 Oct 9; 159 (2): 428-39. https://doi.org/10.1016/j.cell.2014.09.040
-