Starting a new grade, new school or beginning university can be very stressful even without having diabetes, particularly during the COVID pandemic. Many students went to school virtually last year so this autumn may be the first time going to school in person in over a year.
No matter the grade, getting back into the school routine requires a lot of preparation and planning. It is always good to have a plan that includes emergency numbers along with insulin plan info, and how to treat low and high blood sugar levels.
In a perfect world, all school teachers and staff would understand how to manage T1D. Since this isn’t always the case, communication is key. It is important to educate teachers on T1D before school begins. Providing information to the school and classmates on T1D management, especially recognizing the signs of hypoglycemia, will help kids feel comfortable returning to school and importantly will keep them safe and healthy, no matter what the school day brings.
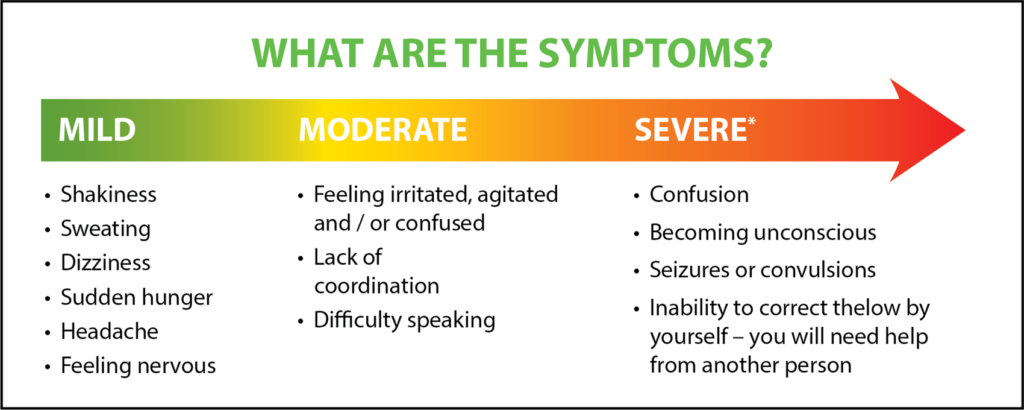
Here’s some information on causes and signs of hypoglycemia.
Low blood sugar can be caused by1:
- Changes to eating patterns such as skipping or delaying meals
- More exercise or activity than usual, or exercising for a long time without eating a snack or adjusting insulin before exercise
- Too much insulin or a change in the time taking insulin
- Stress (presentations, tests, exams, etc.)
It is important always to BE PREPARED!
In case blood sugar levels fall below 4 mmol/L, school staff should be provided in advance with fast-acting glucose (e.g. Dex4 tablets, gels, and liquids). If teachers notice a difference in behaviour related to the signs of low blood sugar or children feel warning signs of hypoglycemia, blood glucose should be checked immediately and treat low blood sugar promptly if needed. If a blood glucose meter is not available but low blood sugar is suspected, treat right away.
Treatment options include1:
- 15 grams of Dex4 glucose tablets (4 tablets), gel (1 pouch), or liquiblast (1 bottle). These are fast-acting and pre-measured
- 3 teaspoons or 3 packets of table sugar dissolved in 15 ml water
- 3/4 cup of juice or soft drink (non diet)
- 1 tablespoon of honey
Afterwards, wait 15 minutes, then check blood sugar again. If blood sugar cannot be tested, monitor the child closely to ensure symptoms of hypoglycemia improve.
As low blood sugar can happen at anytime, it is important to be prepared. Dex4 Glucose products help raise glucose levels FAST.
- Fast-acting
- Pre-measured so you know exactly how much glucose you are consuming
- Fat-Free
- Caffeine, gluten, cholesterol free
- Great-tasting flavours
Additional Resources:
The Diabetes Hope Foundation supports mentorship programs for youth. Buddy systems are available, for more information visit www.diabeteshopefoundation.com/mentor-biographies
1 Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37(suppl 1):S1-S212.
Disclaimer:
Information in this article is provided for informational purposes only and is not a substitute for professional medical advice.